Research Overview
What is AIM?
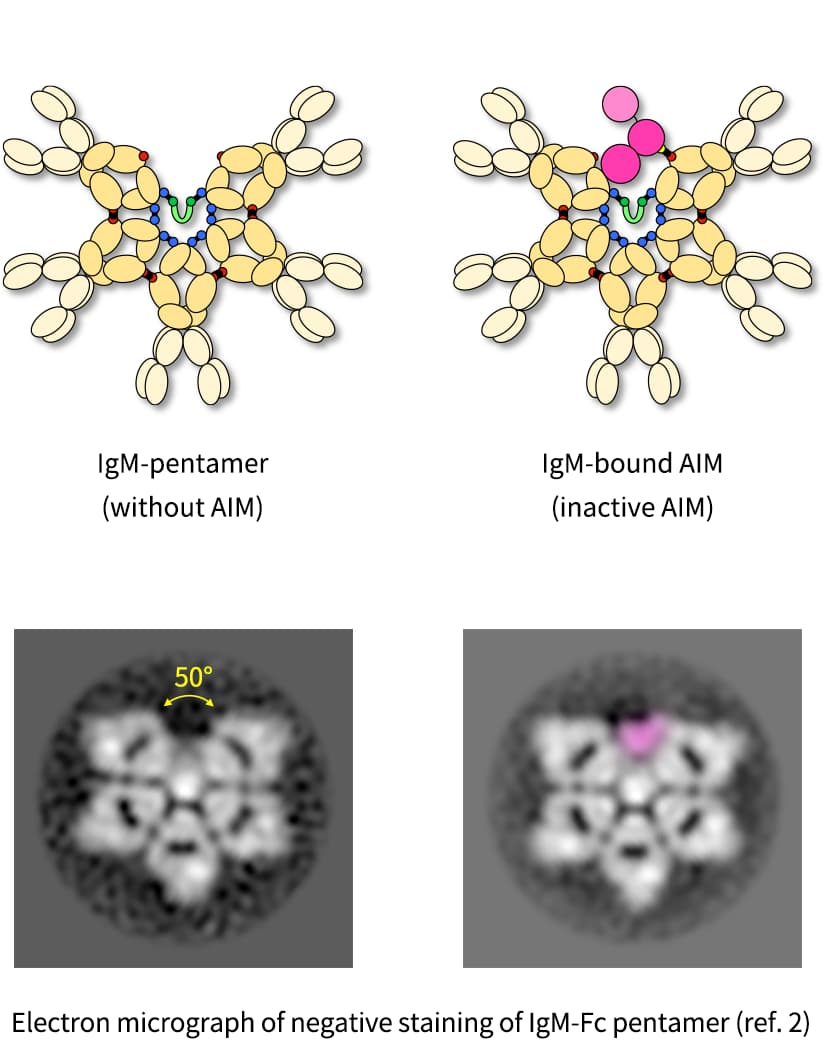
AIM, which stands for Apoptosis Inhibitor of Macrophage, is a blood protein discovered by Dr. Toru Miyazaki and published in 1999 (Ref. 1). In the bloodstream, AIM exists at approximately 5 μg/mL and under healthy condition, AIM is mostly inactive by bound to a type of antibody called Immunoglobulin M (IgM). However, when various "waste" substances, such as cellular debris, damaged proteins, or inflammatory molecules released by dead cells, accumulate in the body, AIM dissociates from IgM and binds to this waste (Ref. 2). The AIM bound to waste acts as a marker, enabling phagocytic cells like macrophages to clean up the waste by engulfing AIM along with it (Ref. 3). This continuous cleaning process protects our bodies from developing diseases. However, if someone has a low level of AIM from birth, if AIM is not functioning properly, or even if they have sufficient AIM but an excessive amount of waste that their AIM cannot handle, waste accumulates in the body, leading to the onset and progression of various diseases. In essence, whether one stays healthy or becomes ill depends on the balance between the amount of waste produced in the body and the ability of AIM and phagocytic cells to clean it up. Therefore, by replenishing insufficient AIM, waste can be adequately eliminated, preventing the onset of many diseases or halting their progression even if someone is already affected. Following this concept, our team has proven through numerous papers (Refs. 4-14) that AIM can prevent and treat various diseases such as kidney disease, stroke, peritonitis, liver cancer, obesity, and fatty liver. We have established a new paradigm for disease treatment, which involves using AIM to enable phagocytic cells to clean up waste in the body, effectively curing many diseases that had no definitive treatments before, like kidney disease and stroke. We are now advancing drug development supported by public organizations, companies, and individuals, aiming to turn AIM into an actual therapeutic agent, in institutions such as the Japan Agency for Medical Research and Development (AMED).
Cats and AIM

Compared to other animals, cats are exceptionally prone to kidney disease, and it is widely known that many cats succumb to this condition. The cause of this ailment has long remained elusive, and like in human kidney disease, there has been no definitive treatment. Our research, published in 2016 (Ref. 15), brought to light a crucial factor behind feline kidney disease: the inability of a cat's AIM to detach from IgM and undertake waste cleanup in the kidneys, resulting in the persistent accumulation of waste and chronic inflammation.
We identified that feline kidney disease is a type of hereditary disorder where AIM does not function properly. Since AIM's malfunction leads to kidney disease, it naturally follows that supplementing AIM should prevent its onset. Building on this insight, we embarked on the development of AIM medicine for cats. Early administration of AIM at regular intervals to facilitate waste cleanup may help prevent kidney disease from developing in the first place. Additionally, even in cases where the disease has progressed, AIM treatment holds the potential to slow down its advancement, with the possibility of long-term kidney function recovery.
The expeditious completion of AIM medicine for cats stands as one of IAM's utmost missions, a testament to our unwavering commitment to the well-being of our feline companions.
Development of
AIM Activating Agents
In addition to directly administering AIM protein to supplement its deficiency, another approach involves enhancing the activation of endogenous AIM that were naturally produced in the body to facilitate waste cleanup. While there is a considerable amount of AIM in the body, not all of it is detached from IgM and activated. The goal is to use medications to release more AIM from IgM, enabling more efficient waste clearance. Directly administering AIM itself is a suitable treatment for acute conditions, such as acute kidney injury or stroke, where the disease worsens rapidly. However, for activating the limited amount of AIM within the body, the amount of waste cleaned up in one instance is constrained. As a result, this approach is believed to be effective in managing chronic diseases with a gradual progression or for preventive measures.
An analogy can be drawn to cleaning a dirty toilet - vigorously scrubbing cleans it instantly, whereas periodically flushing a small amount of water prevents it from getting dirty. In this context, directly administering AIM is akin to vigorous cleaning, while AIM activation represents periodic flushing. AIM activating agents offer a significant advantage as they can be taken orally, unlike AIM itself, which requires injection due to protein breakdown in the gastrointestinal tract.
Furthermore, AIM activation does not necessarily require conventional medications, such as chemical drugs, as natural substances can be used as supplements. In fact, we have identified natural components that activate AIM and are currently developing them as supplements for human consumption. Additionally, these components have shown promising results in activating cat AIM, leading to the development of cat food formulations containing them, with the potential for kidney disease prevention and improvement. At IAM, we are committed to developing AIM activating agents and supplements, harnessing their potential as practical therapeutic and preventive methods for various chronic diseases.
Development of Blood
AIM-based
Diagnostic Techniques

As mentioned earlier, when waste begins to accumulate in the body, AIM detaches from IgM and becomes activated to initiate waste cleanup. If the waste is promptly cleared, the activated AIM is no longer needed and remains bound to IgM. However, when waste removal is inefficient, the body tries to manage the waste, leading to continuous AIM activation. In such situations, there is a higher presence of AIM that has detached from IgM (Free AIM) in the blood. Conversely, elevated levels of Free AIM in the blood suggest that waste removal is not functioning optimally, indicating the initiation of disease.
Exploiting this principle, we are developing diagnostic methods that measure the levels of Free AIM in the blood to detect various diseases at an early stage. By applying this approach, we can identify diseases more sensitively and at an earlier phase than existing diagnostic methods for several conditions (Refs. 16, 17).
References
- Miyazaki T, Hirokami Y, Matsuhashi N, Takatsuka H, Naito M. Increased susceptibility of thymocytes to apoptosis in mice lacking AIM, a novel murine macrophage-derived soluble factor belonging to the scavenger receptor cysteine-rich domain superfamily. J Exp Med. 1999, 189, 413-422.
- Hiramoto E, Tsutsumi A, Suzuki R, Matsuoka S, Arai S, Kikkawa M, Miyazaki T. The IgM pentamer is an asymmetric pentagon with an open groove that binds the AIM protein. Sci Adv. 2018, 4, eaau1199.
- Arai S, Miyazaki T. A scavenging system against internal pathogens promoted by the circulating protein apoptosis inhibitor of macrophage (AIM). Semin Immunopathol. 2018, 40, 567-575.
- Arai S, Kitada K, Yamazaki T, Takai R, Zhang X, Tsugawa Y, Sugisawa R, Matsumoto A, Mori M, Yoshihara Y, Doi K, Maehara N, Kusunoki S, Takahata A, Noiri E, Suzuki Y, Yahagi N, Nishiyama A, Gunaratnam L, Takano T, Miyazaki T. Apoptosis inhibitor of macrophage protein enhances intraluminal debris clearance and ameliorates acute kidney injury in mice. Nat Med. 2016, 22, 183-193.
- Maehara N, Taniguchi K, Okuno A, Ando H, Hirota A, Li Z, Wang C-T, Arai S, Miyazaki T. AIM/CD5L attenuates DAMPs in the injured brain and thereby ameliorates ischemic stroke. Cell Rep. 2021, 36, 109693.
- Kurokawa J, Arai S, Nakashima K, Nagano H, Nishijima A, Miyata K, Ose R, Mori M, Kubota N, Kadowaki T, Oike Y, Koga H, Febbraio M, Iwanaga T, Miyazaki T. Macrophage-derived AIM is endocytosed into adipocytes and decreases lipid droplets via inhibition of fatty acid synthase activity. Cell Metab. 2010, 11, 479-492.
- Maehara N, Arai S, Mori M, Iwamura Y, Kurokawa J, Kai T, Kusunoki S, Taniguchi K, Ikeda K, Ohara O, Yamamura K, Miyazaki T. Circulating AIM prevents hepatocellular carcinoma through complement activation. Cell Rep. 2014, 9, 61-74.
- Hamada M, Nakamura M, Tran MT, Moriguchi T, Hong C, Ohsumi T, Dinh TT, Kusakabe M, Hattori M, Katsumata T, Arai S, Nakashima K, Kudo T, Kuroda E, Wu CH, Kao PH, Sakai M, Shimano H, Miyazaki T, Tontonoz P, Takahashi S. MafB promotes atherosclerosis by inhibiting foam-cell apoptosis. Nat Commun. 2014, 5, 3147.
- Wang C, Yosef N, Gaublomme J, Wu C, Lee Y, Clish CB, Kaminski J, Xiao S, Meyer Zu Horste G, Pawlak M, Kishi Y, Joller N, Karwacz K, Zhu C, Ordovas-Montanes M, Madi A, Wortman I, Miyazaki T, Sobel RA, Park H, Regev A, Kuchroo VK. CD5L/AIM Regulates Lipid Biosynthesis and Restrains Th17 Cell Pathogenicity. Cell. 2015, 163, 1413-1427.
- Arai S, Maehara N, Iwamura Y, Honda S, Nakashima K, Kai T, Ogishi M, Morita K, Kurokawa J, Mori M, Motoi Y, Miyake K, Matsuhashi N, Yamamura K, Ohara O, Shibuya A, Wakeland EK, Li QZ, Miyazaki T. Obesity-associated autoantibody production requires AIM to retain IgM immune complex on follicular dendritic cells. Cell Rep. 2013, 3, 1187-1198.
- Tomita T, Arai S, Kitada K, Mizuno M, Suzuki Y, Sakata F, Nakano D, Hiramoto E, Takei Y, Maruyama S, Nishiyama A, Matsuo S, Miyazaki T, Ito Y. Apoptosis inhibitor of macrophage ameliorates fungus-induced peritoneal injury model in mice. Sci Rep. 2017, 7, 6450.
- A-Gonzalez N, Guillen JA, Gallardo G, Diaz M, de la Rosa JV, Hernandez IH, Casanova-Acebes M, Lopez F, Tabraue C, Beceiro S, Hong C, Lara PC, Andujar M, Arai S, Miyazaki T, Li S, Corbi AL, Tontonoz P, Hidalgo A, Castrillo A. The Nuclear receptor LXRα controls the functional specialization of splenic macrophages. Nat Immunol. 2013, 14, 831-839.
- Kurokawa J, Nagano H, Ohara O, Kubota N, Kadowaki T, Arai S, Miyazaki T. Apoptosis inhibitor of macrophage (AIM) is required for obesity-associated recruitment of inflammatory macrophages into adipose tissue. Proc Natl Acad Sci U S A. 2011, 108, 12072-12077.
- Arai S, Shelton JM, Chen M, Bradley MN, Castrillo A, Bookout AL, Mak PA, Edwards PA, Mangelsdorf DJ, Tontonoz P, Miyazaki T. A role for the apoptosis inhibitory factor AIM/Spalpha/Api6 in atherosclerosis development. Cell Metab. 2005, 1, 201-213.
- Sugisawa R, Hiramoto E, Matsuoka S, Iwai S, Takai R, Yamazaki T, Mori N, Okada Y, Takeda N, Yamamura K-I, Arai T, Arai S, Miyazaki T. Impact of feline AIM on the susceptibility of cats to renal disease. Sci Rep. 2016, 6, 35251.
- Koyama N, Yamazaki T, Kanetsuki Y, Hirota J, Asai T, Mitsumoto Y, Mizuno M, Shima T, Kanbara Y, Arai S, Miyazaki T, Okanoue T. Activation of apoptosis inhibitor of macrophage is a sensitive diagnostic marker for NASH-associated hepatocellular carcinoma. J Gastroenterol. 2018, 53, 770-779.
- Shimizu T, Sawada T, Asai T, Kanetsuki Y, Hirota J, Moriguchi M, Nakajima T, Miyazaki T, Okanoue T. Hepatocellular carcinoma diagnosis using a novel electrochemiluminescence immunoassay targeting serum IgM-free AIM. Clin J Gastroenterol. 2022, 15, 41-51.
Main Research Focus
Pharmaceuticals
for CatsPharmaceuticals
for HumansAIM activation therapy
Diagnosis by AIM
Basic research related to AIM
